Design, delivery, and maintenance of manufacturing support facilities, utilities, process equipment, and automation control so that they perform as intended to meet business objectives, such as capacity, yield, operational efficiency, and reliability. Development of a production process that can repeatedly produce a quality product.. The basic rules in any good manufacturing practice (GMP) regulations specify that the pharmaceutical manufacturer must maintain proper documentation and records. Documentation helps to build up a detailed picture of what a manufacturing function has done in the past and what it is doing now and, thus, it provides a basis for planning what it is.

PPT Importance of Good Manufacturing Practices in the Pharmaceutical

Good Manufacturing Practices Pharmaceutical

Current Good Manufacturing Practices Definition DEFINITION VGF

The GMP Framework Pharmaaccess
Good Manufacturing Practice (GMP) Resources ISPE International

GMP 101 Intro to Good Manufacturing Practice [WEBINAR] YouTube

An Introduction to Good Manufacturing Practice Pharmaceutical and

PPT GOOD MANUFACTURING PRACTICES IN THE PHARMACEUTICAL, BIOTECHNOLOGY

Good Manufacturing Practices GMP in Pharmaceuticals YouTube

GOOD MANUFACTURING PRACTICE GUIDELINE FOR PHARMACEUTICAL INDUSTRY Payhip

Good Manufacturing Practice (GMP) CMC Pharma GmbH

Good Manufacturing practices yield good Quality Good manufacturing

GMP Good Manufacturing Practice Quality Management Certification

Five principles of Good Manufacturing Practice, schematical overview
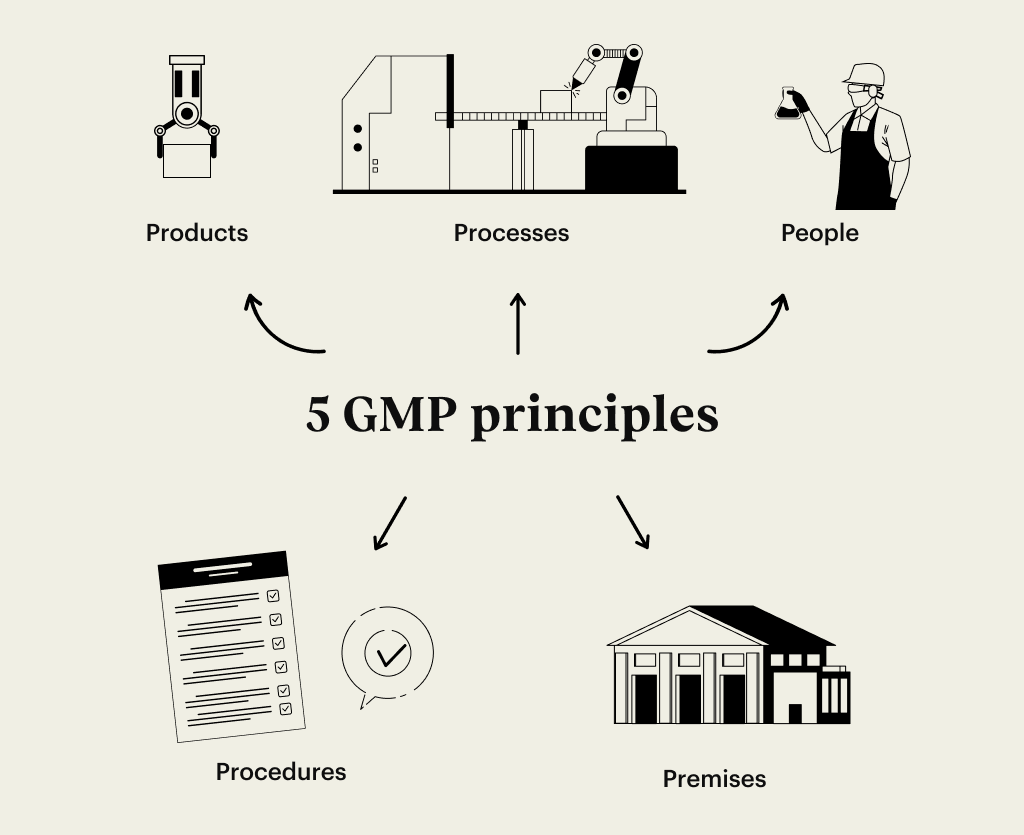
The Complete Guide to Good Manufacturing Practices (GMP) by QVALON QVALON

PPT GMP (Good Manufacturing Practices) Training (183slide PPT

What is good manufacturing practice? Definition and meaning

Figure 2 from A Review on Good Manufacturing Practice (GMP) for
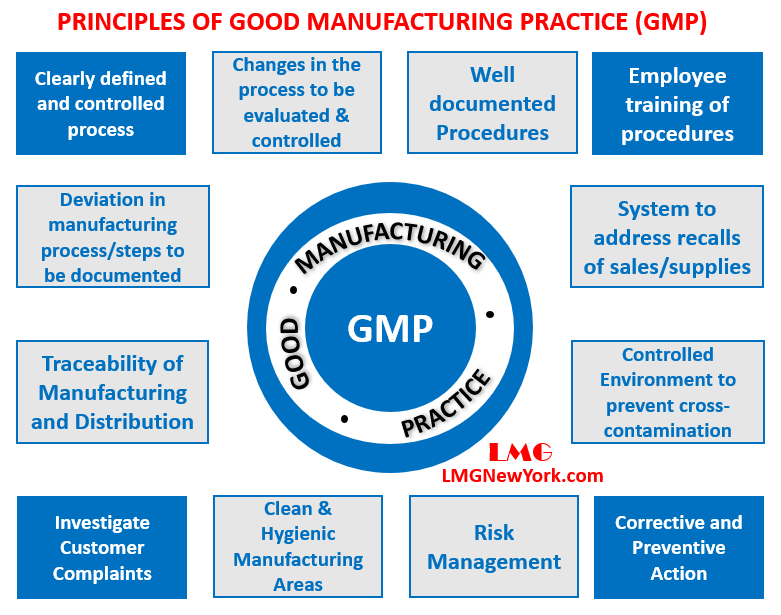
GMP Good Manufacturing Practice LMG New York

Good Manufacturing Practices for Pharmaceuticals
Good manufacturing practice (GMP) is a system for ensuring that products are consistently produced and controlled according to quality standards.. This will improve the health of the individual patient and the community, as well as benefiting the pharmaceutical industry and health professionals. Making and distributing poor quality medicines.. 1It is a prohibited act under section 301 (e) of the FD&C Act to refuse to permit access to or to refuse copying of any record as required by section 704 (a) of the Act. 2See 21 CFR 211.180 (c.